Heat transfer describes the exchange of thermal energy, between physical systems depending on the temperature and pressure, by dissipating heat. The fundamental modes of heat transfer areconduction or diffusion, convection and radiation.
The exchange of kinetic energy of particles through the boundary between two systems which are at different temperatures from each other or from their surroundings. Heat transfer always occurs from a region of high temperature to another region of lower temperature. Heat transfer changes the internal energy of both systems involved according to the First Law of Thermodynamics.[1]
TheSecond Law of Thermodynamics defines the concept of thermodynamic entropy, by measurable heat transfer.
Thermal equilibrium is reached when all involved bodies and the surroundings reach the same temperature. Thermal expansion is the tendency of matter to change in volume in response to a change in temperature.[2]Heat is defined in physics as the transfer of thermal energy across a well-defined boundary around athermodynamic system. The thermodynamic free energy is the amount of work that a thermodynamic system can perform. Enthalpy is a thermodynamic potential, designated by the letter "H", that is the sum of the internal energyof the system (U) plus the product of pressure (P) and volume (V). Joule is a unit to quantify energy, work, or the amount of heat.
Heat transfer is a process function (or path function), as opposed to functions of state; therefore, the amount of heat transferred in a thermodynamic process that changes the state of a system depends on how that process occurs, not only the net difference between the initial and final states of the process.
Thermodynamic and mechanical heat transfer is calculated with the heat transfer coefficient, the proportionalitybetween the heat flux and the thermodynamic driving force for the flow of heat. Heat flux is a quantitative, vectorial representation of the heat flow through a surface.[3]
In engineering contexts, the term heat is taken as synonymous to thermal energy. This usage has its origin in thehistorical interpretation of heat as a fluid (caloric) that can be transferred by various causes,[4] and that is also common in the language of laymen and everyday life.
The transport equations for thermal energy (Fourier's law), mechanical momentum (Newton's law for fluids), and mass transfer (Fick's laws of diffusion) are similar,[5][6]and analogies among these three transport processes have been developed to facilitate prediction of conversion from any one to the others.[6]
Thermal engineering concerns the generation, use, conversion, and exchange of heat transfer. As such, heat transfer is involved in almost every sector of the economy.[7] Heat transfer is classified into various mechanisms, such as thermal conduction, thermal convection, thermal radiation, and transfer of energy by phase changes.
Mechanisms[edit]
The fundamental modes of heat transfer are:
- Advection
- Advection is the transport mechanism of a fluid substance or conserved property from one location to another, depending on motion and momentum.
- Conduction or diffusion
- The transfer of energy between objects that are in physical contact. Thermal conductivity is the property of a material to conduct heat and evaluated primarily in terms of Fourier's Law for heat conduction.
- Convection
- The transfer of energy between an object and its environment, due to fluid motion. The average temperature, is a reference for evaluating properties related to convective heat transfer.
- Radiation
- The transfer of energy from the movement of charged particles within atoms is converted to electromagnetic radiation.
Advection[edit]
By transferring matter, energy—including thermal energy—is moved by the physical transfer of a hot or cold object from one place to another.[8] This can be as simple as placing hot water in a bottle and heating a bed, or the movement of an iceberg in changing ocean currents. A practical example is thermal hydraulics.[citation needed]This can be described by the formula:
where Q is heat flux (W/m²), ρ is density (kg/m³), 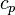 is heat capacity at constant pressure (J/(kg*K)), ΔT is the change in temperature (K),
is heat capacity at constant pressure (J/(kg*K)), ΔT is the change in temperature (K),  is velocity (m/s).
is velocity (m/s).
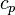 is heat capacity at constant pressure (J/(kg*K)), ΔT is the change in temperature (K),
is heat capacity at constant pressure (J/(kg*K)), ΔT is the change in temperature (K),  is velocity (m/s).
is velocity (m/s).Conduction[edit]
Main article: Thermal conduction
On a microscopic scale, heat conduction occurs as hot, rapidly moving or vibrating atoms and molecules interact with neighboring atoms and molecules, transferring some of their energy (heat) to these neighboring particles. In other words, heat is transferred by conduction when adjacent atoms vibrate against one another, or as electrons move from one atom to another. Conduction is the most significant means of heat transfer within a solid or between solid objects in thermal contact. Fluids—especially gases—are less conductive. Thermal contact conductance is the study of heat conduction between solid bodies in contact.[9]
Steady state conduction (see Fourier's law) is a form of conduction that happens when the temperature difference driving the conduction is constant, so that after an equilibration time, the spatial distribution of temperatures in the conducting object does not change any further.[10] In steady state conduction, the amount of heat entering a section is equal to amount of heat coming out.[9]
Transient conduction (see Heat equation) occurs when the temperature within an object changes as a function of time. Analysis of transient systems is more complex and often calls for the application of approximation theories or numerical analysis by computer.[9]
Convection[edit]
Main article: Convection
The flow of fluid may be forced by external processes, or sometimes (in gravitational fields) by buoyancy forces caused when thermal energy expands the fluid (for example in a fire plume), thus influencing its own transfer. The latter process is often called "natural convection". All convective processes also move heat partly by diffusion, as well. Another form of convection is forced convection. In this case the fluid is forced to flow by use of a pump, fan or other mechanical means.
Convective heat transfer, or convection, is the transfer of heat from one place to another by the movement of fluids, a process that is essentially the transfer of heat viamass transfer. Bulk motion of fluid enhances heat transfer in many physical situations, such as (for example) between a solid surface and the fluid.[11] Convection is usually the dominant form of heat transfer in liquids and gases. Although sometimes discussed as a third method of heat transfer, convection is usually used to describe the combined effects of heat conduction within the fluid (diffusion) and heat transference by bulk fluid flow streaming.[12] The process of transport by fluid streaming is known as advection, but pure advection is a term that is generally associated only with mass transport in fluids, such as advection of pebbles in a river. In the case of heat transfer in fluids, where transport by advection in a fluid is always also accompanied by transport via heat diffusion (also known as heat conduction) the process of heat convection is understood to refer to the sum of heat transport by advection and diffusion/conduction.
Free, or natural, convection occurs when bulk fluid motions (streams and currents) are caused by buoyancy forces that result from density variations due to variations of temperature in the fluid. Forced convection is a term used when the streams and currents in the fluid are induced by external means—such as fans, stirrers, and pumps—creating an artificially induced convection current.[13]
Convection-cooling[edit]
See also: Nusselt number
Convective cooling is sometimes described as Newton's law of cooling:
However, by definition, the validity of Newton's law of cooling requires that the rate of heat loss from convection be a linear function of ("proportional to") the temperature difference that drives heat transfer, and in convective cooling this is sometimes not the case. In general, convection is not linearly dependent on temperature gradients, and in some cases is strongly nonlinear. In these cases, Newton's law does not apply.
Convection vs. conduction[edit]
In a body of fluid that is heated from underneath its container, conduction and convection can be considered to compete for dominance. If heat conduction is too great, fluid moving down by convection is heated by conduction so fast that its downward movement will be stopped due to its buoyancy, while fluid moving up by convection is cooled by conduction so fast that its driving buoyancy will diminish. On the other hand, if heat conduction is very low, a large temperature gradient may be formed and convection might be very strong.
The Rayleigh number ( ) is a measure determining the relative strength of conduction and convection.[citation needed]
) is a measure determining the relative strength of conduction and convection.[citation needed]
 ) is a measure determining the relative strength of conduction and convection.[citation needed]
) is a measure determining the relative strength of conduction and convection.[citation needed]
where
- g is acceleration due to gravity,
- ρ is the density with
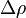 being the density difference between the lower and upper ends,
being the density difference between the lower and upper ends, - μ is the dynamic viscosity,
- α is the Thermal diffusivity,
- β is the volume thermal expansivity (sometimes denoted α elsewhere),
- T is the temperature,
- ν is the kinematic viscosity, and
- L is characteristic length.
The Rayleigh number can be understood as the ratio between the rate of heat transfer by convection to the rate of heat transfer by conduction; or, equivalently, the ratio between the corresponding timescales (i.e. conduction timescale divided by convection timescale), up to a numerical factor. This can be seen as follows, where all calculations are up to numerical factors depending on the geometry of the system.
The buoyancy force driving the convection is roughly 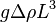 , so the corresponding pressure is roughly
, so the corresponding pressure is roughly 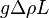 . In steady state, this is canceled by the shear stress due to viscosity, and therefore roughly equals
. In steady state, this is canceled by the shear stress due to viscosity, and therefore roughly equals  , where V is the typical fluid velocity due to convection and
, where V is the typical fluid velocity due to convection and  the order of its timescale.[citation needed] The conduction timescale, on the other hand, is of the order of
the order of its timescale.[citation needed] The conduction timescale, on the other hand, is of the order of 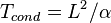 .
.
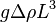 , so the corresponding pressure is roughly
, so the corresponding pressure is roughly 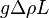 . In steady state, this is canceled by the shear stress due to viscosity, and therefore roughly equals
. In steady state, this is canceled by the shear stress due to viscosity, and therefore roughly equals  , where V is the typical fluid velocity due to convection and
, where V is the typical fluid velocity due to convection and  the order of its timescale.[citation needed] The conduction timescale, on the other hand, is of the order of
the order of its timescale.[citation needed] The conduction timescale, on the other hand, is of the order of 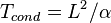 .
.
Convection occurs when the Rayleigh number is above 1,000–2,000.
Thermal radiation occurs through a vacuum or any transparent medium (solid or fluid). It is the transfer of energy by means ofphotons in electromagnetic waves governed by the same laws.[14] Earth's radiation balance depends on the incoming and the outgoing thermal radiation, Earth's energy budget. Anthropogenic perturbations in the climate system, are responsible for a positive radiative forcing which reduces the net longwave radiation loss out to Space.
Thermal radiation is energy emitted by matter as electromagnetic waves, due to the pool of thermal energy in all matter with a temperature above absolute zero. Thermal radiation propagates without the presence of matter through the vacuum of space.[15]
Thermal radiation is a direct result of the random movements of atoms and molecules in matter. Since these atoms and molecules are composed of charged particles (protons and electrons), their movement results in the emission ofelectromagnetic radiation, which carries energy away from the surface.
The Stefan-Boltzmann equation, which describes the rate of transfer of radiant energy, is as follows for an object in a vacuum :
For radiative transfer between two objects, the equation is as follows:
where Q is the heat flux, ε is the emissivity (unity for a black body), σ is the Stefan-Boltzmann constant, and T is the absolute temperature (in Kelvin or Rankine). Radiation is typically only important for very hot objects, or for objects with a large temperature difference.
Radiation from the sun, or solar radiation, can be harvested for heat and power.[16] Unlike conductive and convective forms of heat transfer, thermal radiation can be concentrated in a small spot by using reflecting mirrors, which is exploited in concentrating solar power generation.[17] For example, the sunlight reflected from mirrors heats the PS10 solar power tower and during the day it can heat water to 285 °C (545 °F).[citation needed]
Phase transition[edit]
See also: Phase transition
Phase transition or phase change, takes place in a thermodynamic system from one phase or state of matter to another one by heat transfer. Phase change examples are the melting of ice or the boiling of water. The Mason equation explains the growth of a water droplet based on the effects of heat transport on evaporation and condensation.
Types of phase transition occurring in the four fundamental states of matter, include:
- Solid - Deposition, freezing and solid to solid transformation.
- Gas - Boiling / evaporation, recombination / deionization, and sublimation.
- Liquid - Condensation and melting / fusion.
- Plasma - Ionization.
Boiling[edit]
Main article: Boiling
The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid[19][20] and the liquid evaporates resulting in an abrupt change in vapor volume.
Saturation temperature means boiling point. The saturation temperature is the temperature for a corresponding saturation pressure at which a liquid boils into its vapor phase. The liquid can be said to be saturated with thermal energy. Any addition of thermal energy results in a phase transition.
At low temperatures, no boiling occurs and the heat transfer rate is controlled by the usual single-phase mechanisms. As the surface temperature is increased, local boiling occurs and vapor bubbles nucleate, grow into the surrounding cooler fluid, and collapse. This is sub-cooled nucleate boiling, and is a very efficient heat transfer mechanism. At high bubble generation rates, the bubbles begin to interfere and the heat flux no longer increases rapidly with surface temperature (this is the departure from nucleate boiling, or DNB).
At high temperatures, the hydrodynamically-quieter regime of film boiling is reached. Heat fluxes across the stable vapor layers are low, but rise slowly with temperature. Any contact between fluid and the surface that may be seen probably leads to the extremely rapid nucleation of a fresh vapor layer ("spontaneousnucleation"). At higher temperatures still, a maximum in the heat flux is reached (the critical heat flux, or CHF).
The Leidenfrost Effect demonstrates how nucleate boiling slows heat transfer due to gas bubbles on the heater's surface. As mentioned, gas-phase thermal conductivity is much lower than liquid-phase thermal conductivity, so the outcome is a kind of "gas thermal barrier".
Condensation[edit]
Main article: Condensation
Condensation occurs when a vapor is cooled and changes its phase to a liquid. During condensation, the latent heat of vaporization must be released. The amount of the heat is the same as that absorbed during vaporization at the same fluid pressure.[citation needed]
There are several types of condensation:
- Homogeneous condensation, as during a formation of fog.
- Condensation in direct contact with subcooled liquid.
- Condensation on direct contact with a cooling wall of a heat exchanger: This is the most common mode used in industry:
- Filmwise condensation is when a liquid film is formed on the subcooled surface, and usually occurs when the liquid wets the surface.
- Dropwise condensation is when liquid drops are formed on the subcooled surface, and usually occurs when the liquid does not wet the surface.
- Dropwise condensation is difficult to sustain reliably; therefore, industrial equipment is normally designed to operate in filmwise condensation mode.
Melting[edit]
Main article: Melting
Melting is a physical process that results in the phase transition of a substance from a solid to a liquid. The internal energy of a substance is increased, typically by the application of heat or pressure, resulting in a rise of its temperature to the melting point, at which the ordering of ionic or molecular entities in the solid breaks down to a less ordered state and the solid liquefies. An object that has melted completely is molten. Substances in the molten state generally have reduced viscosity with elevated temperature; an exception to this maxim is the element sulfur, whose viscosity increases to a point due to polymerization and then decreases with higher temperatures in its molten state.[21]
Modeling approaches[edit]
Heat transfer can be modeled in the following ways.
Climate models[edit]
Climate models study the radiant heat transfer by using quantitative methods to simulate the interactions of the atmosphere, oceans, land surface, and ice.
Heat equation[edit]
The heat equation is an important partial differential equation that describes the distribution of heat (or variation in temperature) in a given region over time. In some cases, exact solutions of the equation are available;[22] in other cases the equation must be solved numerically using computational methods.
Lumped system analysis[edit]
Lumped system analysis often reduces the complexity of the equations to one first-order linear differential equation, in which case heating and cooling are described by a simple exponential solution, often referred to as Newton's law of cooling.
System analysis by the lumped capacitance model is a common approximation in transient conduction that may be used whenever heat conduction within an object is much faster than heat conduction across the boundary of the object. This is a method of approximation that reduces one aspect of the transient conduction system—that within the object—to an equivalent steady state system. That is, the method assumes that the temperature within the object is completely uniform, although its value may be changing in time.
In this method, the ratio of the conductive heat resistance within the object to the convective heat transfer resistance across the object's boundary, known as the Biot number, is calculated. For small Biot numbers, the approximation of spatially uniform temperature within the object can be used: it can be presumed that heat transferred into the object has time to uniformly distribute itself, due to the lower resistance to doing so, as compared with the resistance to heat entering the object.[citation needed]
Engineering[edit]
Heat transfer has broad application to the functioning of numerous devices and systems. Heat-transfer principles may be used to preserve, increase, or decrease temperature in a wide variety of circumstances.[citation needed] Heat transfer methods are used in numerous disciplines, such as automotive engineering, thermal management of electronic devices and systems, climate control, insulation, materials processing, and power station engineering.
Insulation, radiance and resistance[edit]
Thermal insulators are materials specifically designed to reduce the flow of heat by limiting conduction, convection, or both.Thermal resistance is a heat property and the measurement by which an object or material resists to heat flow (heat per time unit or thermal resistance) to temperature difference.
Radiance or spectral radiance are measures of the quantity of radiation that passes through or is emitted. Radiant barriers are materials that reflect radiation, and therefore reduce the flow of heat from radiation sources. Good insulators are not necessarily good radiant barriers, and vice versa. Metal, for instance, is an excellent reflector and a poor insulator.
The effectiveness of a radiant barrier is indicated by its reflectivity, which is the fraction of radiation reflected. A material with a high reflectivity (at a given wavelength) has a low emissivity (at that same wavelength), and vice versa. At any specific wavelength, reflectivity = 1 - emissivity. An ideal radiant barrier would have a reflectivity of 1, and would therefore reflect 100 percent of incoming radiation. Vacuum flasks, or Dewars, are silvered to approach this ideal. In the vacuum of space, satellites usemulti-layer insulation, which consists of many layers of aluminized (shiny) Mylar to greatly reduce radiation heat transfer and control satellite temperature.[citation needed]
Devices[edit]
- Heat engine is a system that performs the conversion of heat or thermal energy to mechanical energy which can then be used to do mechanical work.[23][24]
- Thermocouple is a temperature-measuring device and widely used type of temperature sensor for measurement and control, and can also be used to convert heat into electric power.
- Thermoelectric cooler is a solid state electronic device that pumps (transfers) heat from one side of the device to the other when electrical current is passed through it. It is based on the Peltier effect.
- Thermal diode or thermal rectifier is a device that causes heat to flow preferentially in one direction.
Heat exchangers[edit]
Main article: Heat exchanger
A heat exchanger is used for more efficient heat transfer or to dissipate heat. Heat exchangers are widely used inrefrigeration, air conditioning, space heating, power generation, and chemical processing. One common example of a heat exchanger is a car's radiator, in which the hot coolant fluid is cooled by the flow of air over the radiator's surface.[citation needed]
Common types of heat exchanger flows include parallel flow, counter flow, and cross flow. In parallel flow, both fluids move in the same direction while transferring heat; in counter flow, the fluids move in opposite directions; and in cross flow, the fluids move at right angles to each other. Common constructions for heat exchanger include shell and tube, double pipe, extruded finned pipe, spiral fin pipe, u-tube, and stacked plate.[further explanation needed]
A heat sink is a component that transfers heat generated within a solid material to a fluid medium, such as air or a liquid. Examples of heat sinks are the heat exchangers used in refrigeration and air conditioning systems or the radiator in a car. A heat pipe is another heat-transfer device that combines thermal conductivity and phase transition to efficiently transfer heat between two solid interfaces.
Examples[edit]
Architecture[edit]
Efficient energy use is the goal to reduce the amount of energy required in heating or cooling. In architecture, condensation and air currents can cause cosmetic or structural damage. An energy audit, can help to assess the implementation of recommended corrective procedures. For instance, insulation improvements, air sealing of structural leaks or the addition of energy-efficient windows and doors.[25]
- Smart meter is a device that records electric energy consumption in intervals.
- Thermal transmittance is the rate of transfer of heat through a structure divided by the difference in temperature across the structure. It is expressed in watts per square meter per kelvin, or W/m²K. Well-insulated parts of a building have a low thermal transmittance, whereas poorly-insulated parts of a building have a high thermal transmittance.
- Thermostat is a device to monitor and control temperature.
Climate engineering[edit]
See also: Anthropogenic heat
Climate engineering consist of carbon dioxide removal and solar radiation management. Since the amount ofcarbon dioxide determines the radiative balance of Earth atmosphere, carbon dioxide removal techniques can be applied to reduce the radiative forcing. Solar radiation management is the attempt to absorb less solar radiation to offset the effects of greenhouse gases.
Greenhouse effect[edit]
The greenhouse effect is a process by which thermal radiation from a planetary surface is absorbed by atmospheric greenhouse gases, and is re-radiated in all directions. Since part of this re-radiation is back towards the surface and the lower atmosphere, it results in an elevation of the average surface temperature above what it would be in the absence of the gases.
Heat transfer in the human body[edit]
See also: Wet-bulb temperature
The principles of heat transfer in engineering systems can be applied to the human body in order to determine how the body transfers heat. Heat is produced in the body by the continuous metabolism of nutrients which provides energy for the systems of the body.[26] The human body must maintain a consistent internal temperature in order to maintain healthy bodily functions. Therefore, excess heat must be dissipated from the body to keep it from overheating. When a person engages in elevated levels of physical activity, the body requires additional fuel which increases the metabolic rate and the rate of heat production. The body must then use additional methods to remove the additional heat produced in order to keep the internal temperature at a healthy level.
Heat transfer by convection is driven by the movement of fluids over the surface of the body. This convective fluid can be either a liquid or a gas. For heat transfer from the outer surface of the body, the convection mechanism is dependent on the surface area of the body, the velocity of the air, and the temperature gradient between the surface of the skin and the ambient air.[27] The normal temperature of the body is approximately 37 °C. Heat transfer occurs more readily when the temperature of the surroundings is significantly less than the normal body temperature. This concept explains why a person feels “cold” when not enough covering is worn when exposed to a cold environment. Clothing can be considered an insulator which provides thermal resistance to heat flow over the covered portion of the body.[28] This thermal resistance causes the temperature on the surface of the clothing to be less than the temperature on the surface of the skin. This smaller temperature gradient between the surface temperature and the ambient temperature will cause a lower rate of heat transfer than if the skin were not covered.
In order to ensure that one portion of the body is not significantly hotter than another portion, heat must be distributed evenly through the bodily tissues. Blood flowing through blood vessels acts as a convective fluid and helps to prevent any buildup of excess heat inside the tissues of the body. This flow of blood through the vessels can be modeled as pipe flow in an engineering system. The heat carried by the blood is determined by the temperature of the surrounding tissue, the diameter of the blood vessel, the thickness of the fluid, velocity of the flow, and the heat transfer coefficient of the blood. The velocity, blood vessel diameter, and the fluid thickness can all be related with the Reynolds Number, a dimensionless number used in fluid mechanics to characterize the flow of fluids.
Latent heat loss, also known as evaporative heat loss, accounts for a large fraction of heat loss from the body. When the core temperature of the body increases, the body triggers sweat glands in the skin to bring additional moisture to the surface of the skin. The liquid is then transformed into vapor which removes heat from the surface of the body.[29] The rate of evaporation heat loss is directly related to the vapor pressure at the skin surface and the amount of moisture present on the skin.[27]Therefore, the maximum of heat transfer will occur when the skin is completely wet. The body continuously loses water by evaporation but the most significant amount of heat loss occurs during periods of increased physical activity.
Cooling techniques[edit]
Evaporative cooling[edit]
Evaporative cooling happens when water vapor is added to the surrounding air. The energy needed to evaporate the water is taken from the air in the form of sensible heat and converted into latent heat, while the air remains at a constant enthalpy. Latent heat describes the amount of heat that is needed to evaporate the liquid; this heat comes from the liquid itself and the surrounding gas and surfaces. The greater the difference between the two temperatures, the greater the evaporative cooling effect. When the temperatures are the same, no net evaporation of water in air occurs; thus, there is no cooling effect.
Laser cooling[edit]
In Quantum Physics laser cooling is used to achieve temperatures of near absolute zero (−273.15 °C, −459.67 °F) of atomic and molecular samples, to observe unique quantum effects that can only occur at this heat level.
- Doppler cooling is the most common method of laser cooling.
- Sympathetic cooling is a process in which particles of one type cool particles of another type. Typically, atomic ions that can be directly laser-cooled are used to cool nearby ions or atoms. This technique allows cooling of ions and atoms that cannot be laser cooled directly.[citation needed]
Magnetic cooling[edit]
Main articles: Magnetic refrigeration and Magnetic evaporative cooling
Magnetic evaporative cooling is a process for lowering the temperature of a group of atoms, after pre-cooled by methods such as laser cooling. Magnetic refrigeration cools below 0.3K, by making use of the magnetocaloric effect.
Radiative cooling[edit]
Radiative cooling is the process by which a body loses heat by radiation. Outgoing energy is an important effect in the Earth's energy budget. In the case of the Earth-atmosphere system, it refers to the process by which long-wave (infrared) radiation is emitted to balance the absorption of short-wave (visible) energy from the Sun. Convective transport of heat and evaporative transport of latent heat both remove heat from the surface and redistribute it in the atmosphere.
Thermal energy storage[edit]
Thermal energy storage refers to technologies used to collect and store energy for later use. They can be employed to balance energy demand between day and nighttime. The thermal reservoir may be maintained at a temperature above (hotter) or below (colder) than that of the ambient environment. Applications include later use in space heating, domestic or process hot water, or to generate electricity.













No comments:
Post a Comment